In the Externalized BioPharma Industry, Seamless CollaborationDrives Life-Altering Medicines and Therapies to Patients Faster
- maurinabignotti
- Jul 30, 2025
- 11 min read
June 2024

By 20/15 Visioneers Team
Background
Today’s biopharma collaboration or “externalization” cannot be efficiently achieved without proper scientifically aware software. In the externalized biopharma industry, seamless collaboration is crucial for accelerating the delivery of life-altering medicines and therapies to patients. By integrating efforts across various stakeholders, the industry can enhance efficiency, innovation, and patient outcomes. The term "collaboration," first coined by Charles Reade in the 1860s, refers to cooperative efforts but can also imply potentially treacherous cooperation with adversaries. In R&D, particularly with Contract Research Organizations (CROs), collaboration has both tactical and strategic dimensions. Tactical partnerships are program/project-based, with CROs competing to provide services to biopharmaceutical sponsors. In contrast, strategic partnerships adopt a consultative approach, where sponsors and CROs work together to bring products to market, see Figure 1. This partnership leverages the CRO’s subject-matter expertise and integrates it with the sponsor’s strategic objectives. Activities within strategic partnerships include business development, market surveillance, and marketing strategies, as well as managing clinical trials, budgets, timelines, and regulatory submissions. This approach fosters more integrative and collaborative environment, enhancing the efficiency and effectiveness of the drug development process, and providing added value through improved business development, marketing insights, and strategic marketing initiatives.
_____________________________________
“A paradigm shift in the early 2000s saw large biopharmaceutical companies mandating R&D externalization to CROs, with predictions by ~2010 that 80% of R&D would be externalized.” John F. Conway
_____________________________________
In the early 2000s, a significant shift took place in the biopharmaceutical industry as major companies increasingly turned to Contract Research Organizations (CROs) to handle their Research and Development (R&D) processes, seeking greater efficiency and flexibility. By 2010, this trend had gained momentum, with forecasts indicating that around 80% of R&D activities would be outsourced to CROs by large pharmaceutical firms. Today, this strategy has become standard practice, with numerous companies heavily reliant on CROs for various facets of their R&D endeavors, marking a profound and enduring shift in the industry's operational landscape. The contract research organization (CRO) services market size was valued at USD 82.60 billion in 2023 and is projected to grow USD 188.52 billion by 2030, exhibiting a CAGR of 12.5% during 2023-2030 [1].

Before the pandemic in 2020, numerous biopharmaceutical companies strategically organized their operations by partnering with Clinical or Contract Research Organizations (CROs). This strategy evolved from the desire for cost-effectiveness and the benefits of accessing specialized expertise, faster timelines, adaptable resource allocation, geographical advantages, advanced technologies, and talent pools. This shift towards utilizing CROs for discovery research has spurred the emergence of virtual startups and enhanced capital allocation efficiency for smaller biopharmaceutical firms. With bolstered in-house capabilities, CROs have assumed a crucial role in drug discovery and development, exemplified by Charles River Laboratories' transformation from a provider of animal testing services to a multinational CRO with extensive capacities tailored to the requirements of major biopharmaceutical companies [2]. In 2022, the CRO data management services segment accounted for its highest growth rate. It is projected to grow further at a CAGR of 12.5% with a need for improved data quality and integrity and sustainable ways for data analytics to meet ever-improving quality standards. Scientific and Clinical Data Management is naturally gaining importance due to not only its much-needed role in the streamlined and uninterrupted development of drugs and medical devices but the reuse of FAIR data in AI efforts [3].
Since 2020, with the onset of the COVID-19 pandemic, strategic efforts for rapid asset discovery and development have been accelerated, emphasizing the critical importance of efficient collaboration within the biopharmaceutical sector. Biopharmaceutical sponsors are expanding collaborative networks including CROs, academia and leading research institutions for fast-paced growth. Biopharmaceutical sponsors acknowledged the necessity of concerted research endeavors, prompting the growth of collaborative networks beyond conventional Contract Research Organizations (CROs) to encompass academic and numerous leading research institutions. This trend highlights the increasing need for seamless coordination among diverse stakeholders within the industry, as reported by Fierce Biotech [4]. Please refer to Figure 2.

The Pursuit of Continual Improvement
Outsourcing collaboration with Contract Research Organizations (CROs) in research and development (R&D) has emerged as a linchpin for the success of medium and large biopharmaceutical companies, consistently delivering vital services either at a reduced cost or comparable to internal operations. For smaller or startup organizations, CROs have offered quick and reliable nimble services. Despite encountering occasional challenges, dedicated individuals adeptly navigate complexities, often resolving issues without significant increases in manpower. This steadfast commitment to problem-solving, bolstered by advancements in communication, technology integration, and lean methodologies, has cultivated stronger and more efficient partnerships between companies and their outsourced collaborators.
__________________________________________
“Companies seeking cost reductions, improved quality, and faster R&D timelines are increasingly opting to outsource certain activities.”
__________________________________________
The strategic decision by department heads to persist with R&D outsourcing, notwithstanding higher initial costs, underscores the organization's unwavering dedication to continuous improvement. This forward-thinking approach seeks to refine processes and operations over time, ensuring sustained competitiveness and fostering a culture of innovation. Consequently, and as mentioned above with Charles River, CROs have evolved into formidable service providers, prioritizing operational efficiency over reliance on blockbuster drugs for profitability.
While project/program sponsors retain ownership of creativity and intellectual property, the prospect of milestone payments or profit-sharing arrangements serves as a catalyst for collaborative efforts and breakthrough innovations. This mutually beneficial relationship not only enhances operational efficiency but also drives advancements in the biopharmaceutical industry, positioning both companies and CROs for ongoing success and expansion in the dynamic landscape of R&D outsourcing.
Collaboration is Getting More Complex in an Already High Paced Environment
Contract Research Organizations (CROs) play a pivotal role in advancing drug and therapy discovery through collaborative efforts, operating globally across organizational boundaries. As illustrated in Figure 3, outsourcing certain aspects of research projects can accelerate processes such as technology innovation, intentional FAIR data management, quality assurance, and compliance, while also reducing stress among team members in a dynamic R&D environment.

Currently, extracting and disseminating information through web portals, emails, and presentations hinders innovation and quick adjustments. This highlights the increasing need for cloud-capable scientific software solutions like ELNs, LIMS, CDS, etc. These digital and sustainable FAIR data tools facilitate efficient planning, execution, and documentation of scientific experiments, enabling swift and seamless sharing of details and data. This also allows internal and external collaborators, including institutes, partners, and CROs, to coordinate changes effectively in their respective areas of responsibility, and most importantly, protect intellectual property rights.
One crucial aspect of drug discovery and market introduction is preserving intellectual property while ensuring relevant information remains accessible. This process demands significant effort, time, and resources. Partnering with a CRO can expedite this process costeffectively, aiding in idea protection, hypothesis formulation, experiment planning, technology development, and downstream analysis. Utilizing the latest innovative and sustainable technologies, can standardize processes within teams and effectively address human errors related to communication, task prioritization, progress tracking, and onboarding/off-boarding of both internal and external teams.
Challenges in Collaboration
The timelines for success in biopharma are often demanding and necessitate a holistic approach to outsourcing, encompassing both people and technology. While some costs, like those for SaaS (Software as a Service) solutions, are transparent, the project's overall "soft" costs—such as process development, project/program management, coordination delays and handling errors—are often hidden or difficult to quantify. We have outlined common collaboration challenges in Table 1 below.

Given the complexity of drug discovery and the critical race to market, failing to consider the impact of a CRO can have dire consequences on the programs. A recent report from Syneos Health® shows analysis of more than 15 years of NME innovation reveals that internally developed drugs are, on average, 40% more commercially successful than externally Organizational challenges for success Technology challenges for success Clear governance for data and issues Silos of data and poor metadata Clear definitions for data, QC, analysis, and decisions Manual communication for data access Clear understanding of end-to-end decision Modular system that is flexible enough for complicated data Prioritizing and communicating work Onboarding and off-boarding CRO/CDMO resources with a high flux of users Tracking outsourced projects Preserving a sponsor’s intellectual property without limiting relevant information Standardizing and integrating external data for comprehensive downstream analytics Conflict resolution and capturing decisions developed comparators [5]. Additionally, a report from McKinsey estimates that communication time costs exceed $75,000 per year per department, with most of this time spent on external communication [6]. These 2 examples are perfect examples of how inefficiencies in larger companies and the inability to improve externalization and collaboration processes can have a negative effect.
Identifying the right CRO early can benefit an organization by helping to define program goals and key checkpoints, create appropriate business models, select the right clients and partners, preserve intellectual property, and use specific results and metrics to identify improvements or determine when to sunset a program without excessive time and resource investment. This approach ensures that FAIR data processes are utilized from the start, intellectual property rights are protected, knowledge transfer is maintained, and alignment with market demands and evolving regulations is achieved. Moreover, it guarantees that experiments and results are reproducible. Failing in these aspects can lead to wasted time, resources and money, slow down decision-making processes, and ultimately result in program failure, which was outlined by both Syneos Health® and McKinsey in their analysis.
_________________________________________
“Biopharmaceutical sponsors are driving the expansion of collaborative networks beyond traditional CROs to enhance concerted research efforts.”
_________________________________________
Efficient Collaboration Drives Operational Excellence
For an R&D program to succeed, it must develop innovative solutions that accelerate the identification and treatment of diseases or drugs. As we have been discussing, the cornerstone of this success is efficient collaboration. As discussed, collaboration involves internal and external teams within the organization such as partners, sponsors, and CROs. Both types of collaboration significantly impact the success of an R&D program. Focusing specifically on external collaborations like contract research, organizations can drive fit-forpurpose solutions and efficient data exchange by delivering:
Insight: Collective expertise through effective and efficient collaboration
Flexibility: Dynamic scaling of contract partners to meet evolving business needs
Control: Visibility into project timelines allows sponsors to optimize efficiency and resource use as well as accelerate drug innovation
Data Capture and Collaboration: Cloud-native ELNS (electronic lab notebooks) and LIMS for biologists and chemists to collaborate, capture, organize, share, and explore data
Accuracy: Eliminate errors in manual data wrangling
Communication: Facilitate communication while protecting intellectual property
Harmonization: Automated transformation of unstructured reports into structured data empowers fast, comprehensive, streamlined analytics, visualization, and AI/ML
____________________________________
“Empower your drug discovery programs with streamlined collaboration and transformative ROI through strategic CRO management.”
____________________________________
Managing the Business is an Imperative that Sometimes gets Deprioritized
An efficient collaboration platform can provide a competitive edge where being the first to market is crucial for success. The Return on Investment (ROI) from managing the relationship with the CRO on a program or project can be transformative. Sponsoring organizations benefit from improved cost efficiency and productivity, as the CRO has a clearer understanding of project stages, aiding in approvals and payments. This enhanced transparency mitigates risks quickly by minimizing delays and errors. Furthermore, better communication builds stakeholder trust and allows for greater focus on critical issues. This relationship effectively requires CRO collaboration software with these capabilities:
Administration: Simplified project initiation and project termination, user onboarding, and security setup
2. Drug/Therapy Design: Ideation workspace for capturing drug designs and hypotheses
3. Intellectual Property Protection: Built-in masking of proprietary codes, properties, and material IDs
4. Project Management: Scientifically-minded tools for tracking collaboration progress
5. Data Exchange: Automated transformation of unstructured CRO, CMO, and CDMO reports into structured data ready for analytics and visualization
By adopting a software platform with these capabilities, you will accelerate various processes by reducing administrative burdens and expediting project timelines. This streamlining of processes not only improves cost-efficiency and productivity but also ensures smoother project initiation, management, and completion. Furthermore, the platform enhances the Data Capture and Collaboration: Cloud-native ELNS (electronic lab notebooks) and LIMS for biologists and chemists to collaborate, capture, organize, share, and explore data Accuracy: Eliminate errors in manual data wrangling Communication: Facilitate communication while protecting intellectual property Harmonization: Automated transformation of unstructured reports into structured data empowers fast, comprehensive, streamlined analytics, visualization, and AI/ML 1. Administration: Simplified project initiation and project termination, user onboarding, and security setup 2. Drug/Therapy Design: Ideation workspace for capturing drug designs and hypotheses 3. Intellectual Property Protection: Built-in masking of proprietary codes, properties, and material IDs 4. Project Management: Scientifically-minded tools for tracking collaboration progress 5. Data Exchange: Automated transformation of unstructured CRO, CMO, and CDMO reports into structured data ready for analytics and visualization overall effectiveness and reliability of the partnership between the sponsor and the CRO by fostering better communication, transparency, and trust, leading to more successful and timelier project outcomes. The project culture improves dramatically!
______________________________________
“Enhance Collaboration Efficiency and Strategic Advantage in Sponsor-CRO Partnerships through Comprehensive Platform Solutions.”
______________________________________
Examples of Problems Solved
Effective communication is the key to success. Here we describe some challenges and how a CRO can help overcome them efficiently:
1. A common challenge many groups face is keeping colleagues and the organization updated on their work. To address this, a CRO can develop solutions such as project dashboards, resource allocation, work assignments, and milestones and checkpoints to enhance transparency in the workspace.
2. Another challenge is ensuring results are reproducible and capturing all data points for filing intellectual property (IP) rights. With the assistance of a CRO, experts can archive all data points using FAIR data standards and develop reports and plots that follow industry standards, effectively overcoming this issue.
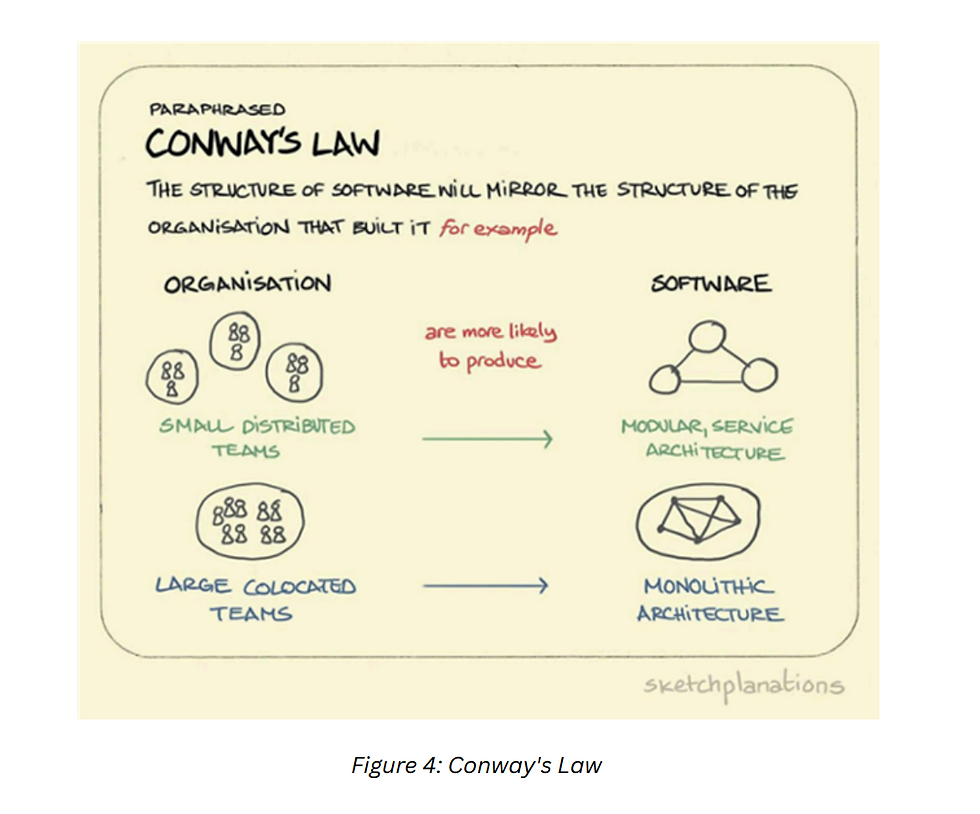
We described two main scenarios above, but there are many other ways organizations have been utilizing CROs to achieve success. By eliminating redundant and time-consuming processes such as collaborator onboarding/off-boarding and project/program management, a CRO helps foster a healthy, sustainable culture. This support enables institutions to develop unique and innovative technologies, from bench research to data analytics. Conway’s principle illustrates how next-generation scientific collaboration can set the foundation for new, creative approaches to success in highly collaborative environments, see Figure 4. By following this principle, organizations can achieve success more quickly, eliminating gaps in different project areas and driving business forward.
Current Companies Meeting the Described Collaboration Needs
Revvity Signals (www.revvitysignals.com) provides exceptional collaborative solutions and experiences for drug discovery. The company streamlines complex processes, enhances user experience, and accelerates innovation by offering expert support and advanced solutions for organizational success. Their comprehensive suite of tools is designed to optimize drug discovery workflows and facilitate seamless collaboration across teams.
One of their standout solutions, Signals Synergy for streamlining CRO collaboration, offers real-time project tracking and cloud-based capabilities for experiment planning, tracking, documentation, data capture, IP protection and data exchange for downstream analytics. This platform allows researchers to efficiently manage and document their experiments in a collaborative environment, ensuring that sponsor and CRO members have access to the most up-to-date information. The cloud-based infrastructure also provides robust data security and scalability, accommodating the needs of both small and large research teams.
Revvity Signals’ solutions are particularly beneficial in the context of precision medicine, where rapid and informed decision-making is crucial. By leveraging their advanced tools and expert support, organizations can enhance their research capabilities, streamline clinical trials, and bring innovative therapies to market more quickly and effectively. This holistic approach improves drug development efficiency and enhances clinical outcomes, ultimately benefiting patients and healthcare providers.
Conclusion
The collaborative relationship between a sponsor and a CRO is becoming common in the biopharma industry to expedite drug development research and bring it to market. These outsourced collaborations play an important role in leveraging deep expertise in new biologics and cutting-edge technologies, making the process more efficient and effective. This trend allows sponsors to tap into the specialized skills and advanced infrastructure of CROs, which often possess the latest technological innovations and scientific expertise. Additionally, CROs can provide regulatory knowledge and operational support, ensuring that clinical trials are conducted in compliance with global standards, thus accelerating the approval process. By leveraging the strengths of CROs, biopharma companies can focus more on their core competencies, such as discovery and early-stage research, while the CRO handles the complexities of clinical development and regulatory submissions.
As the future of precision medicine evolves, it is crucial to implement technologies that foster a collaborative culture. This involves integrating platforms and tools that enable seamless communication and data sharing among various stakeholders, including researchers, clinicians, and regulatory bodies. Advanced technologies such as cloud-based systems, artificial intelligence, and machine learning can significantly enhance the ability to analyze large datasets, identify patterns, and make informed decisions swiftly.
Outsourcing vendors with expertise in the emerging needs of rapid decision-making should be prioritized in the evaluation process. These vendors can provide specialized services that cater to the dynamic requirements of precision medicine, including adaptive trial designs, real-time data analytics, and personalized treatment strategies. By leveraging their expertise, biopharma companies can ensure that they remain agile and responsive to the fast-paced developments in this field. This approach not only improves the efficiency of drug development but also accelerates the delivery of tailored therapies to patients, thereby enhancing clinical outcomes, overall healthcare quality and quality of life for patients.
In this paper, we discussed how choosing the right CRO can benefit an organization by putting them on the fast track with their expertise in various aspects of research, thereby driving business value. If you have any further questions, please reach out to 20/15 Visioneers team and let us help you through the process.
References
2. History. 2024. electronic www.criver.com/about-us/about-us-overview/our- story/ourhistory.
3. CRO Industry Report.
4. Fierce Biotech. "Is R&D collaboration the key to a brighter future in biopharma?" https://www.fiercepharma.com/sponsored/rd-collaboration-key-brighter-futurebiopharma-0. 2024. electronic.
5. Syneos Health. "Internal R&D Versus Externalization: Which Pharma Strategy Yields Greater Success?" www.syneoshealth.com/insights-hub/internal-rd-versusexternalization- which-pharma-strategy-yields-greater-success. 2023. electronic.
6. McKinsey. n.d. McKinsey Quarterly 2006 Number 3.




Comments