Can Carbon Fullerenes Help Us Trap Nuclear Waste? 🧪
- maurinabignotti
- Aug 8, 2025
- 2 min read
Updated: Nov 21, 2025
By Alexander Goldberg, Ph.D.
Visiting Senior Materials Science Visioneer
A new angle on nuclear waste management through carbon nanomaterials and molecular modeling.
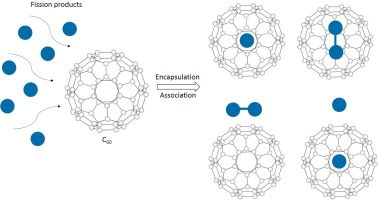
As the global nuclear energy landscape evolves, decommissioning of nuclear reactors has emerged as a critical task—not only from a technical standpoint but also from environmental and policy perspectives. One of the most persistent challenges is the long-term storage of radioactive isotopes, especially cesium-137 (Cs-137). As an example, old types of reactors that undergo decommissioning are those that contain coolant circuits composed of sodium and water in separate loops. In some accidents, the mixing of water and sodium can result in water-sodium explosions, posing significant safety concerns. One critical issue is the contamination of sodium coolant with radioactive cesium-137. To address this, cesium traps have been developed [See: Romanenko et al., Nuclear Technology 150, 79–99 (2005)].
Cs-137 is a fission product with a 30-year half-life, emitting beta radiation radiation as it decays into a radioactive isotope of barium (Ba-137m) which subsequently decays into a stable isotope (Ba-137). Cs-137 mobility in the environment and biological systems makes it one of the most hazardous components of nuclear waste. Effective capture and immobilization of this isotope is essential for safe reactor shutdown and waste containment.
One material showing promise in this area is Reticulated Vitreous Carbon (RVC) — a highly porous, glassy form of carbon with a large surface area. RVC has been used in cesium traps to remove Cs from primary reactor coolant (often sodium). But its amorphous structure is still the subject of scientific debate.
Interestingly, recent studies suggest that RVC may incorporate fragments of fullerene-like structures, such as incomplete C₆₀ cages. These nanocarbon motifs are known for their high surface reactivity, which could enhance cesium adsorption. This raises an intriguing possibility: could fullerene-based or fullerene-like materials be engineered as next-generation cesium traps? [See: Harris, P.J.F., J. Mater. Sci. 48, 565–577 (2013)]
By functionalizing C₆₀—for example, substituting carbon atoms with electron-donating or electron-withdrawing groups—we might significantly increase Cs binding affinity. Computational chemistry offers tools to simulate these interactions and predict how cesium ions bind to different carbon surfaces. This opens a path to rationally design materials with tailored adsorption properties for nuclear waste remediation.
The broader implication is clear: advanced carbon nanomaterials, guided by molecular modeling, may offer scalable, tunable solutions to one of the most persistent problems in nuclear waste management.
As researchers, engineers, and policymakers, we should continue to explore the intersection of materials science, computational modeling, and nuclear safety.




Comments